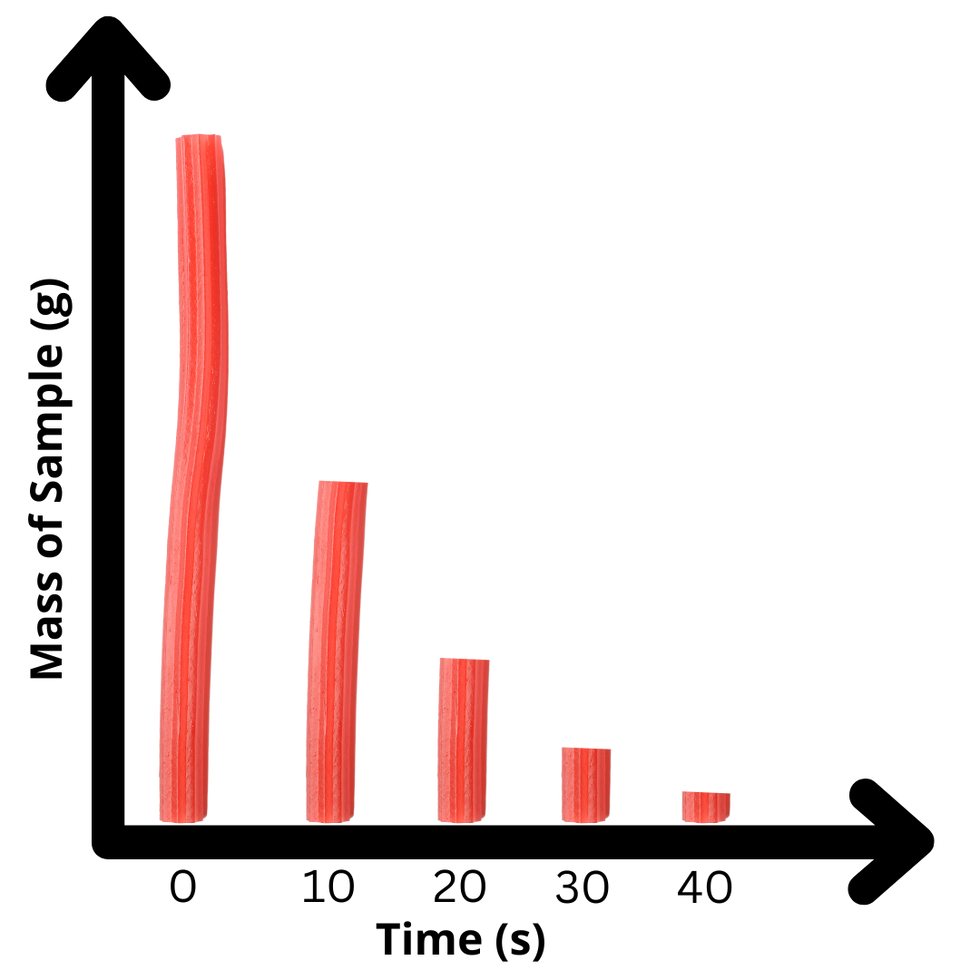
Twizzler Decay: Modeling Radioactive Decay & Half-Life
In this lab, we’ll model half-life using Twizzlers as our "radioactive atoms." With each "cut," you’ll simulate the decay process, observing how the sample decreases step by step. By the end, you’ll see how half-life works and understand the predictability of radioactive decay over time.
Radioactive decay is a natural process where certain unstable atoms release energy to become more stable. These atoms, known as radioisotopes, give off particles or energy as they break down. This breakdown rate is specific to each radioactive element; some elements decay quickly, while others take thousands or even millions of years to change.
To understand this process, we use the concept of half-life. A half-life is the time it takes for half of the atoms in a radioactive sample to decay. Imagine it as a countdown—every half-life period, half of the remaining radioactive atoms "disappear." In the example graph, you can see how a 10-gram sample of a radioactive substance decays over 50 days. The substance loses half of its mass, from 10 grams to 5 grams, in 15 days. So, the half-life for this substance is 15 days.
In this lab, we’ll model half-life using Twizzlers as our "radioactive atoms." With each "cut," you’ll simulate the decay process, observing how the sample decreases step by step. By the end, you’ll see how half-life works and understand the predictability of radioactive decay over time.